For each molecule on the worksheet, the Lewis Dot Structure, the number of valence electrons, the electron arrangement (E.A.) and the molecular geometry (M.G.) are given, respectively. Click on the rotating molecule for summary of geometries. PF5. 40 electrons tbp tbp.In the PF 5 Lewis structure Phosphorus (P) is the least electronegative so it goes in the center. In the Lewis structure for PF 5 there are a total of 40 valence Drawing the Lewis Structure for PF5. Viewing Notes: PF5 is similar to PCl5 and PBr5. If you can do those Lewis structures PF3 will be easy.Lewis Structures. Drawing each bond in a molecule as two dots gets old very fast. To save time chemists usually depict a bond as a line Alternative Representations of Organic Structures. Exercise 6 Complete Table 2 by supplying the molecular formula for each of the bond line diagrams shown....EXCEPTIONS 3. Lewis dot structure 4. Formal Charge: 5. Resonance: Isomers: 4. Types of Bonds: -Single vs. double vs.triple bonds: -Bond Strength: -Bond Length: 5. VSEPR Model 6. Polarity of Molecules Rules for writing Lewis dot structures for molecules: 1. Write the skeletal structure of.Structure of Pf5.. As a result they will be pushed apart giving the pf5 molecule a trigonal bipyramid molecular geometry or shape. the pf5 bond angle The way to determine the molecular geometry of SeF4 is to first draw the Lewis Dot Structure. I can't draw it out on the website, but if you were to do...
pf5 lewis structure - Bing
For the PF5 Lewis structure we first count the valence electron... Hi, this is Dr. B. Let's do the Lewis structure for PF5. On the periodic table, Phosphorus is in group 5, it has 5 valence electrons.Phosphorus Pentafluoride, PF5. Lewis and Three-Dimensional Structures. Trigonal Bipyramid.Lewis Structure by Bond Determination. 1. Know how to determine the valence electron for all elements. 2. (Connectivity) From the Chemical Complete Lewis structure by drawing atomic connectivity. Write bonds in the structure and the place remaining electrons to selected atoms in the...These files are related to Pf5 lewis structure shape . Just preview or download the desired file. for each atom in each molecule and add it to the Lewis Structure; d) fill in the bipyramidal. Molecular geometry: bent.

Drawing Lewis Electron Dot Structures
The Lewis structure indicates that each Cl atom has three pairs of electrons that are not used in bonding (called lone pairs) and one shared pair of electrons (written between the atoms). Writing Lewis Structures with the Octet Rule. For very simple molecules and molecular ions, we can write...Draw lewis structure of PF5. Draw lewis structure of XeF2.A Lewis structure shows the placement of the valence electrons around each of the atoms in a molecule. They are important because they allow us to predict the shapes of molecules, the types and relative strengths of the bonds, and regions of high and low electron density within the molecule.Chemistry learning made easy.This tutorial will help you deal with the lewis structure and moleculargeometry for phosphorus pentafluoride (PF5).Category:Lewis structures. From Wikimedia Commons, the free media repository. Jump to navigation Jump to search. estructura de Lewis (es); Lewis egitura (eu); Estructura de Lewis (ast); структура Льюиса (ru); Elektronenformel (de); Lewis structure (en-gb); ساختار لوویس (fa); 路易士結構 (zh)...
Error
This document was once not discovered on this server.We hope the hyperlinks at the left and those underneath shall be helpful in helping you in finding the ideas you are after:
Please touch the webmanager should you require additional lend a hand or to document an issue similar to a dead link.Geometry of Molecules | Everything have Molecules

The Shapes Of Molecules

ClF5 Lewis Structure - How to Draw the Lewis Structure for ...

Estructura De Lewis Para Pcl5 - 2020 idea e inspiración

What is the structure of PF5, and how can we explain its ...
32 Electron Dot Diagram For Phosphorus - Wiring Diagram List
Christman AP Chemistry Unit 6: Bonding and Molecular Geometry

Science Archives - Geometry of Molecules

Pentafluorure de phosphore — Wikipédia

PF5 Lewis structure, Molecular Geometry, Bond angle and ...
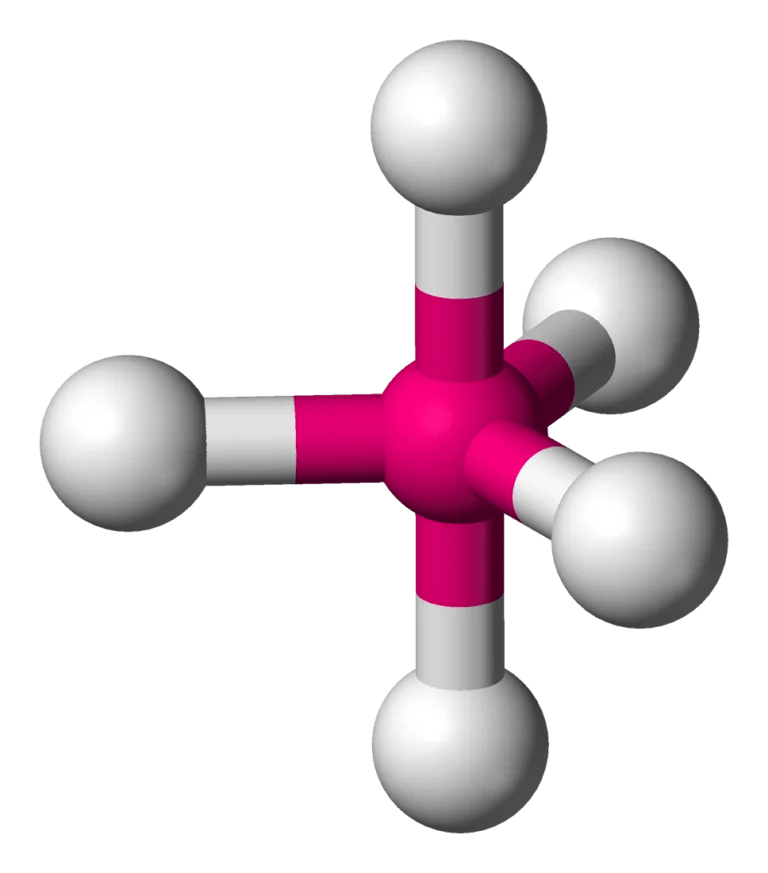
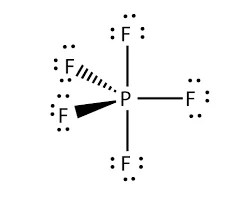
PF5 Lewis structure, Molecular Geometry, Bond angle and ...
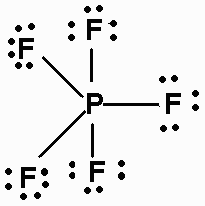
34 Lewis Dot Diagram For Phosphorus - Wiring Diagram List
akdjhfadklhjkhdfhj

PF5 Molecular Geometry / Shape and Bond Angles | Chemistry ...

What are the limitations of the octet rule? + Example

Phosphorpentafluorid - Wikipedia

Lewis Structure For Pf5 видео :: WikiBit.me

Octahedral Molecular Geometry

Lewis Dot Diagram For Phosphorus - Free Wiring Diagram

Vsepr Shape of So3 Trigonal Planar Shaped Molecule

Chemistry 1E03-01: Tutorials
0 comments:
Post a Comment