In the Lewis structure for ClO2- we put Chlorine (Cl) at the center of the structure since it is the least electronegative. There are total of 20 valence electrons for the ClO2- Lewis structure. Remember that the negative sign counts as one valence electron.Chlorite | ClO2- | CID 197148 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safetyOur videos prepare you to succeed in your college classes. Let us help you simplify your studying. If you are having trouble with Chemistry, Organic, Physics, Calculus, or Statistics, we got your back! Our videos will help you understand concepts, solve your homework, and do great on your exams.These try to depict the electronic structure of ClO^- anion. In fact the given diagram is highly misleading and ambiguous. The valence electrons around the chlorine atom were depicted. Why should only the chlorine-based electrons be? As written, the chlorine atom is neutral. A similar depiction should have been given around oxygen, and here a formal negative charge would be associated with theQuestion: Complete The Lewis Structure Of The Chlorite Ion, ClO2, Which Is Used As A Bleaching Agent. Complete Only The Two Resonance Structures In Which The Formal Charges Are Closest To Zero. You Do Not Need To Enclose The Lewis Structure In Square Brackets.
Chlorite | ClO2- - PubChem
The ClO2 Lewis structure has 19 valence electrons meaning that there will be an odd number of valence electrons in the structure. For the Lewis structure for ClO2 you should take formal charges into account to find the best Lewis structure for the molecule.The LibreTexts libraries are Powered by MindTouch ® and are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739.The ClO2 Lewis structure has 19 valence electrons meaning that there will be an odd number of valence electrons in the structure. Typically, ionic Lewis dot structures include the ionic charge, so the Na ion is labeled +1 and Cl is labeled -1. share. entry.parameters["potcar_symbols"] = ['PAW_PBE Fe_pv 06Sep2000', A Lewis Dot Structure can beThanks for A2A. Question : What is the structure of ClO2? Pic credit : Google images. Uses of Chlorine dioxide : 1. It is used as disinfectant in treatment of water. 2. Used as antimicrobial agent in water that are used in processing poultry, wash...

Solution: Draw the lewis structure for ch... | Chemistry
Get the free "Lewis structure" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha.Therefore, P = 6n + 2 - V = 6 * 3 + 2 - 19 = 1 So there is only 1 π electron in ClO 2 So the structure of Step 1 is the Lewis structure. Electrons are placed around each atom so that the octet rule is obeyed.Clo2 Lewis Structure. Source(s): https://shorte.im/a9blc. 0 0. Still have questions? Get your answers by asking now. Ask Question + 100. Join Yahoo Answers and get 100 points today. Join. Trending Questions. Trending Questions. Pure magnesium metal is often found as ribbons and can easily burn in the presence of oxygen. ?Nitrogen Dioxide (NO 2) is a covalent compound that is composed of a central nitrogen atom single bonded to an oxygen atom and a double bond with another oxygen atom. At room temperatures, nitrogen dioxide is a reddish-brown gas that has a density of 1.8 g/dm 3.It is slightly toxic to humans, on account of its tendency to react in the human body and produce reactive species of nitrogen andFor the Lewis structure for ClO 2 place Chlorine (Cl) in the center of the structure since it is the least electronegative. ClO 2 has an odd number of valence electrons (19). That means that the Chlorine (Cl) atom will have an unpaired electron and therefore won't have an octet (it will have seven in ClO 2.
Commonly Tested Lewis Structures
We draw Lewis Structures to predict:
-the form of a molecule.
-the reactivity of a molecule and how it might interact with other molecules.
-the bodily properties of a molecule such as boiling point, surface tension, and many others.
Video: Drawing the Lewis Structure for ClO2
The ClO2 Lewis structure has 19 valence electrons which means that there shall be an abnormal selection of valence electrons in the structure. For the Lewis structure for ClO2 you should take formal fees into account to find the most productive Lewis structure for the molecule.
For the ClO2 Lewis structure, calculate the entire choice of valence electrons for the ClO2 molecule. After figuring out how many valence electrons there are in ClO2, place them across the central atom to complete the octets.
Note that Chlorine is the least electronegative and is going on the heart of the ClO2 Lewis structure.
[embedded content]It is helpful for those who:
Try to attract the ClO2 Lewis structure sooner than observing the video. Watch the video and spot if you happen to missed any steps or knowledge. Try buildings similar to ClO2 for extra practice. List of Lewis StructuresChemistry Net: 11/01/2012 - 12/01/2012

Clf5 Polar Or Nonpolar

35 MCQs On Practice Exam 4 For Chemistry Fundamentals I | CHM 2045 - Docsity

CHAPTER 7 - COVALENT BONDING - Nthurston.k12.wa.us 7: COVALENT BONDING You Should Understand The Nature Of The Covalent Bond. Atoms In A Given Formula And These Have Nothing To

A Common Form Of Elemental Phosphorus Is T... | Clutch Prep

Lewis Structure Exercise - Academic Computer Center Pages 1 - 8 - Flip PDF Download | FlipHTML5

Solved: B. The Lewis Diagram For CIO, Is: :0-0-0 The Elect... | Chegg.com

CH 101 Discussion Worksheet #8 Fall 2020 1 Answers To Discussion Worksheet 8 Large Group Discussion 1. (a) Orbitals Are 3D
Sr(cn)2 Lewis Structure

What Is The Structure Of [CpMoCl2] 2? - Quora
7 - Wiley

Lewis Structure Covalent Compounds Calculator
Solved: Lewis Diagrams -- Triatomic Molecules And Ions Of | Chegg.com
SI Units/Dimensional Analysis
Lewis Structures | Atomic | Interaction

Clo3 Formal Charge
Fall 2001 Exam 1 Answers
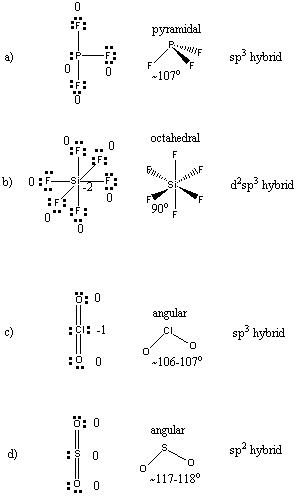
Solved: Q.12. Write The Lewis Structure And Predict The Sh... | Chegg.com

0 comments:
Post a Comment