So the species present, let's do this species present. When any H s support dissolves in water, we will have some What is taking me so long? I'm doing voiceovers that like 100 more voiceovers to dio I'll obviously have any h s so forth. Oh, I wrote the equation first equals any plus h s +04 and then the H...Dissolved oxygen is the presence of these free O2 molecules within water. The bonded oxygen molecule in water (H2O) is in a compound and Light can penetrate water, though the depth that it can reach varies due to dissolved solids and other light-scattering elements present in the water.Pollution of water occurs when substances that will modify the water in negative fashion are Water is a natural solvent, enabling most pollutants to dissolve in it easily and contaminate it. Such an oil spill can cause varying damage to species in the ocean, depending on the amount of oil spill, the...Answer: the major species present when C₃H₆ (OH) ₂ is the same molecule. Explanation: When solutes dissolve in water there are two possibilities The compound C₃H₆ (OH) ₂ (propylene glycol) is a covalent compound, so when dissolved in water it will not dissociate but will remain as a molecule...Crystals dissolve in water because the heat of solution of the ions is enough to overcome the lattice B A substance that increases the amount of hydroxide ions present when it is dissolved in water. Consider the following reaction: PbCl2 + 2 NaOH → Pb(OH)2 + 2 NaCl What would be the major...
How dissolved oxygen affects fish behaviour
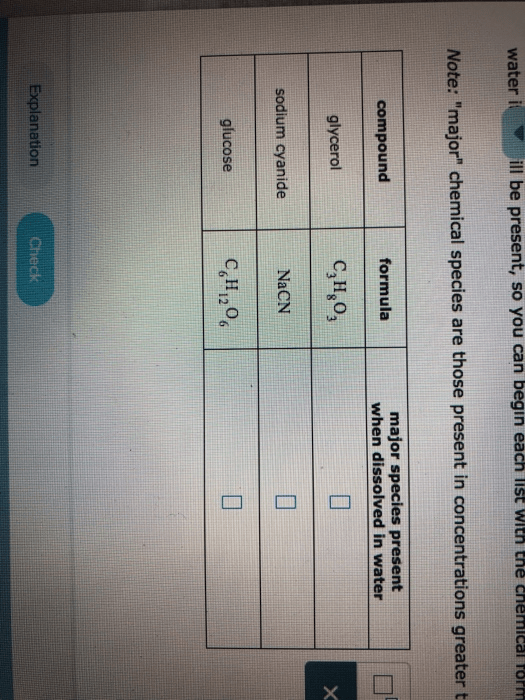
This depends on the composition of the species present in the solution. The substance that has the highest composition will be the major species.Total Dissolved Solids (TDS) in water are some organic and inorganic materials, which include minerals and ions that are dissolved in a particular TDS present in water is not a measure of any single contaminant and so is generally not regulated as a health issue by many government agencies.Answer: the major species present when C₃H₆(OH)₂ is the same molecule. The compound C₃H₆(OH)₂ (propylene glycol) is a covalent compound, so when dissolved in water it will not dissociate but will remain as a molecule, and that molecules is the species solvated by the molecule of water.When a compound like NaCl dissolves in water, it dissociates completely, so that the main speceies not NaCl formulas, but ions of Na⁺ and Cl⁻, not of the molecules.That leads to the answer: the majority of the species present when acetone is dissolved in water the molecules of acetone...

Sources and Causes of Water Pollution That Affect Our Environment...
When an ionic compound dissolves in water, the cation and the anion are separated from each other by these layers of water. So we can say that sodium chloride is soluble in water. Some species, particularly ionic compounds, dissolve but have a limit to their solubility.issolved oxygen - oxygen molecules dissolved in water - is a major indicator of water quality. Like the air we breathe, the survival of aquatic life depends on a sufficient level of oxygen dissolved in water. When it drops below levels necessary for sustaining aquatic life, it becomes a significant water...3) When dissolved, covalent bonded compounds remain as separate molecules, then it is said that the major species present in the solution is the molecule. That leads to the answer: the major species present when acetone is dissolved in water is the molecules of acetone (you do not need to state...Total dissolved solids (TDS) is a measure of the dissolved combined content of all inorganic and organic substances present in a liquid in molecular, ionized, or micro-granular (colloidal sol) suspended form. TDS concentrations are often reported in parts per million (ppm).Glucose(C6H12O6) is itself major species. SO glucose and H2O major species. FeBr3ionises to Fe+view the full answer.
Yahoo Answers has close down as of May 4, 2021. Yahoo Answers was once once a key a part of Yahoo's products and services, but it has declined in recognition through the years as the needs of our contributors have modified. We made up our minds to shift our assets clear of Yahoo Answers to focus on merchandise that better serve our contributors and deliver on Yahoo's promise of offering top rate depended on content.
As of May 4, 2021 you'll no longer access the website online, however you can still request a obtain of your Yahoo Answers information until June 30, 2021. To lend a hand you with this transition now we have compiled a list of questions that can come up throughout this procedure.
Will this impact my Yahoo Account or different Yahoo services and products?No, those adjustments are particular to Yahoo Answers. They would possibly not have an effect on your Yahoo Account or another Yahoo services.
Where must I am going when I've questions in the future?Yahoo Search can be utilized to search out answers and information from the internet. Our Yahoo COVID page provides data and sources concerning the Coronavirus pandemic.
Can I obtain my Yahoo Answers content? What content is available to me?Your Yahoo Answers knowledge obtain will go back all user-generated content material, together with your questions, solutions and images. You won't be able to download different users' content, questions, or answers.
Do I have to obtain my content?No, the content download is optional. However, if you choose to download your content, you've to take action before June 30, 2021.
When will I get my Yahoo Answers content material?Our group works as rapid as conceivable to make data to be had, but it might probably take as much as 30 days to obtain your content download.
I downloaded my Yahoo Answers content material, how do I view it?Your content can be formatted in JSON (JavaScript Object Notation) and may well be laborious to appear thru at a look. We have resources on viewing and managing your account information that permit you to understand your data obtain.
How can I percentage my comments/comments about this transformation?Send any comments or feedback you've gotten referring to this decision to yahoo_answers_sunset@verizonmedia.com. Thanks for taking the time to percentage your thoughts with us.
ser/toN70P.WANYC-7X MBWVPW ARSME O CHEMICAL HRACTIONS ...

Solved: The Chemical Formulae Of Some Acids Are Listed In ...

Solved: The Names And Chemical Formulae Of Some Chemical C ...

Yimin HUANG | Postdoctoral research fellow | Yunnan ...

Major species present when dissolved in water ammonium ...

Number of species and percentage composition of major taxa ...

Chemistry Archive | July 07, 2017 | Chegg.com
Chemistry Archive | December 22, 2016 | Chegg.com

plz answer question and show work muia for water (H,U). e ...

Rajani PANCHANG | Assistant Professor | M.Sc. (Geol.); PhD ...

Solved: Water I Ill Be Present, So You Can Begin Each List ...
PETER PEDUZZI | Prof. Dr. | University of Vienna, Vienna ...

compound formula major species present when dissolved in ...

ALEKS - Standardizing a Base Solution by Titration - YouTube

Chemistry Archive | August 31, 2017 | Chegg.com
Solved: Each Chemical Compound Are Soluble In Water, When ...

Solved: Some Chemical Compounds Are Listed In The First Co ...

Yimin HUANG | Postdoctoral research fellow | Yunnan ...

Botdf Idgaf Download

Solved: = Knowledge Check Question 6 Alma The Names And Ch ...

Solved: The Names And Chemical Formulae Of Some Chemical C ...

0 comments:
Post a Comment